Wilson’s disease (WD) (synonym hepatolenticular degeneration), an autosomal recessive disorder of copper metabolism, results in copper deposition within liver, brain and other organs with the age of onset of 15-21 years ( range 5 to 35). The consequent neurological involvement predominantly results in combinations of tremor, dystonia and Parkinsonism, with associated neuropsychiatric symptomatology.
The disorder is rare in the over 50 age group.
Early diagnosis is essential, as treatment may reverse symptoms and prevent progression. Diagnosis depends on the demonstration of
Low serum caeruloplasmin
Excess urinary copper excretion
Presence of corneal Kayser-Fleischer rings (seen in virtually every case of neurological Wilson’s disease).
There is a wide spectrum of clinical manifestations, but the most important and basic symptoms of the disease can be divided into hepatic, neurologic, and psychiatric manifestations.
Genetics/Inheritance
Mutations affect the function of the ATP7B gene which encodes a copper transporter, ATPase7B, involved in transport of copper in the trans-Golgi network in hepatocytes as well as biliary copper excretion.
There are more than 500 known pathogenic mutations in the ATP7B gene: in Europe and the United States, H1069Q is the commonest mutation. There appears to be genotype-phenotype correlation; the H1069Q mutation causes only a partial defect of ATP-ase7B and leads to milder disturbances of copper metabolism with a later onset of neurologic symptoms. By contrast, frameshift and nonsense mutations correlate with more severe impairment of copper metabolism and an earlier onset of clinical symptoms. A substantial proportion of individuals with Wilson disease have compound heterozygous mutations, with distinct mutations on each copy of the gene.
Pathogenesis
Altered protein function leads to marked accumulation of copper in the liver, with resultant mitochondrial impairment and ultimately to cirrhosis. Stored copper is then released into the blood, leading to its accumulation in other tissues (brain, kidney, cornea). An excess of free copper is toxic for most tissues, leading to secondary damage due to damage to mitochondria, respiratory cycle defects, and release of copper into the cytoplasm.
In the brain, copper toxicosis leads to neuronal degeneration and astrocyte changes, probably due to hyperammonemia (in the course of Krebs cycle failure) with the occurrence of pathologic astrocytes.
Clinical Features
1. The hepatic presentation
The hepatic presentation of WD occurs almost 10 years earlier than neurologic symptoms, with the mean age of onset 11.4 year, although the condition has been diagnosed in infancy.
• Asymptomatic elevation of liver enzymes (usually asymptomatic elevation of serum aminotransferases, often found accidentally).
• Acute hepatitis
• Acute liver failure. This is most often seen in young women, and is usually accompanied by severe haemolytic anaemia, renal failure, and encephalopathy. It is one of the main indications for liver transplantation in WD treatment.
• Chronic hepatitis with liver cirrhosis (decompensated or compensated) is the most frequent presentation of WD; cirrhosis may develop without clinical symptoms and can be discovered accidentally when neurologic signs occur.
2. The neurologic presentation
This occurs in about half of patients and has a mean age of onset of 20 years (range 6-72 years).
In many cases it is difficult to classify the patients’ neurologic symptoms because of mixed presentations. The most commonest neurologic presentations are:
-Tremor
Tremor is the most frequent neurologic symptom. “Wing beating tremor” is one of the characteristic signs and consists of a proximal tremor of high amplitude: present when the shoulders are abducted and the arms flexed at the elbows. However, this is not the commonest tremor manifestation. Tremor phenomenology is varied and a wide range of tremors may occur, including kinetic, resting, postural, and intention tremors; tremors that are symmetric or asymmetric; of variable amplitude; and those that are intermittent whereas others are constant and progressive.
-Dysarthria is common and may be classified as dystonic, cerebellar, parkinsonian, or mixed.
-Parkinsonism 40% of patients. Rigidity and bradykinesia are typically present.
-Dystonia
Dystonia, occurring in 10% to 30% of cases, is usually severe and resistant to treatment. It may be either focal (risus sardonicus, orofacial dystonia, hand dystonia, tongue dystonia), segmental (trunk dystonia), or generalized dystonia.
-Ataxia
Other movement disorders, (chorea and ballism), as well as autonomic system impairment, are less frequent.
Signs of pyramidal dysfunction and sensory disturbances are rare. They are sometimes caused by treatment complications (eg, myeloneuropathy due to copper deficiency caused by “overtreatment”).
3. Psychiatric manifestations
Psychiatric manifestations can be the initial presentation in a quarter of patients, and two thirds of patients develop psychiatric symptoms during course of the illness.
These include:
Cognitive impairment.
This is typically found in patients with severe neurological impairment. In children, decline in school performance can occur; whereas in older patients, impairment of executive functions and visuospatial processing is a common finding, rarely leading to dementia.
Social and other phobias, and obsessive compulsive disorder
Change in personality. This often includes abnormal behaviour, irritability, aggressiveness, and disinhibition.
Mood disorders and psychosis
4. Ophthalmologic manifestations
These include Kayser-Fleischer ring and sunflower cataracts. Both are pathognomonic, but reversible during treatment. Their identification is therefore helpful for the diagnosis and monitoring of treatment.
The Kayser-Fleischer ring is caused by copper deposition in Descemet’s membrane of the cornea. It is a brown discoloration seen at the limbus of the cornea. This occurs in 90% to 100% of patients with neurologic presentation, but in only half of patients with hepatic presentation and in a quarter of asymptomatic patients.
There are rare medical conditions with eye changes similar to the K-F ring, including primary biliary cirrhosis, cryptogenic cirrhosis, cholestatic cirrhosis, as well as neoplastic disorders with high serum copper levels (multiple myeloma, leukaemia) and high-dose oestrogen treatment.
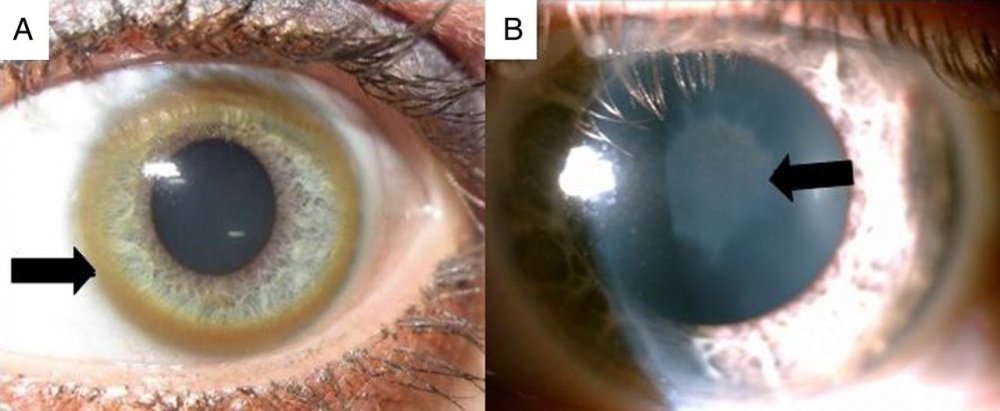
Sunflower cataract is caused by copper deposits located directly under the anterior lens capsule, and resembles a sunflower (central disc with radiating petals).
Haematological changes
Thrombocytopenia, leukopenia, haemolytic anaemia
Bone and joint involvement
Pain, osteoporosis, spontaneous fractures
Renal involvement
Hypercalciuria, hyperphosphaturia, nephrocalcinosis
Investigations
1. Serum caeruloplasmin level
The serum caeruloplasmin level is decreased to less than 2.83 μmol/L (normal range: 2.83-5.50 μmol/L) in almost all patients. A moderate decrease of caeruloplasmin is observed in about 20% of carriers with a mutation.
False negatives (normal or even increased serum caeruloplasmin, may be seen in patients with decompensated liver cirrhosis or during oestrogen therapy.
False-positive results (decreased level of caeruloplasmin) may be seen in aceruloplasminemia, sprue, protein deficiency states including protein-losing enteropathy, nephrotic syndrome, and malnutrition.
Note that the caeruloplasmin level also depends on the method of examination (the immunologic assay often overestimates the protein level, whereas the nephelometric method is more accurate).
2. The total serum copper level
Total serum copper is not a reliable diagnostic test because it includes not only caeruloplasmin-bound copper (low in Wilson disease, proportional to low caeruloplasmin) but also free copper, which is elevated. Consequently, the total serum copper level may be normal or elevated, particularly if there is acute liver failure, which causes free copper to be released into the serum. The total serum copper level in patients is usually decreased to less than 10 μmol/L (normal range: 10-22 μmol/L) and it mostly represents the copper bound to caeruloplasmin (decreases with caeruloplasmin). Free, non- caeruloplasmin bound copper (calculated manually as difference between total and caeruloplasmin -bound serum copper) is increased to greater than 15 mg/dL in untreated patients.
Free copper is more relevant for diagnosis and treatment. The false-negative, extremely high total serum copper levels in patients with WD occur in acute liver failure with haemolytic anaemia. Increased total copper level also can be observed in some neoplastic disorders (eg, leukaemia, multiple myeloma) and during oestrogen intake.
Normal free seurm copper is: 1.6-2.4 μmol/L
3. Daily urinary copper excretion
Daily urinary copper excretion is the best single test and is typically increased to greater than 0.8 μmol/24 hours (normal range: 0.3-0.8 μmol/24 hours). Values over 0.4 μmol/24 hours may be significant.
False-negative results may be seen, especially in children and asymptomatic cases. False-positive results were observed in patients with acute liver failure and neoplastic disorders, such as leukaemia or multiple myeloma.
4. Brain imaging
Typical MRI changes include symmetrical hyperintense or mixed intensity T2- weighted and FLAIR signal change in the striatum, especially the putamen, and also the thalamus, midbrain and pons.
The cerebellum, corticospinal tracts, and subcortical areas (atrophy in 70% of cases) are also affected.
Classical radiological signs include:
Face of the giant panda sign (referring to the combination of high signal intensity in the tegmentum except for the red nucleus (eyes) with preservation of signal intensity of the lateral portion of the pars reticulata of the substantia nigra (ears) and hypointensity of the superior colliculus (mouth).
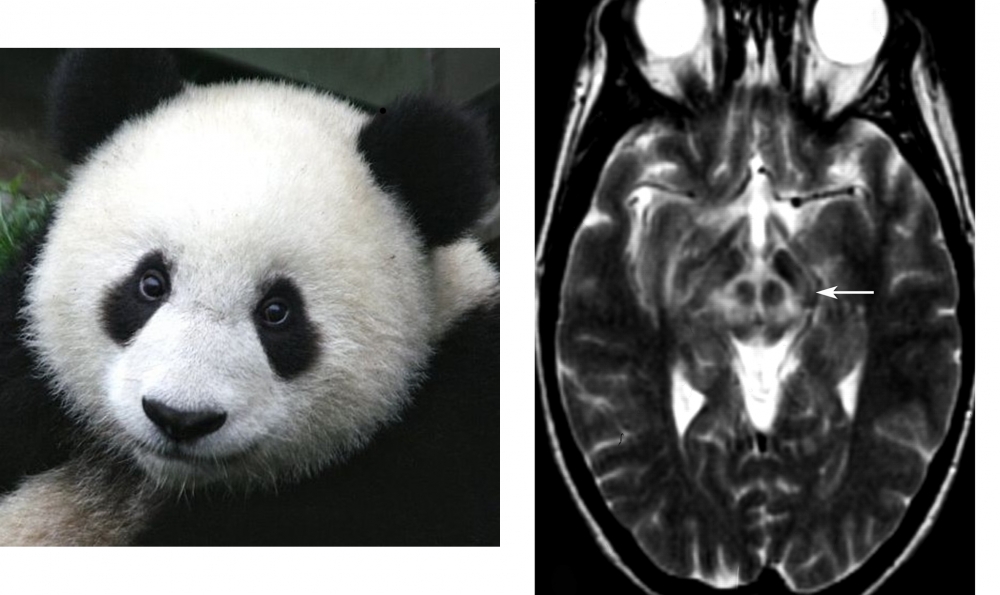
Case courtesy of Assoc Prof Frank Gaillard, Radiopaedia.org. From the case rID: 36073; Jacobs DA, Markowitz CE, Liebeskind DS, Galetta SL. The "double panda sign" in Wilson's disease. Neurology. 2003 Oct 14;61(7):969. doi: 10.1212/01.wnl.0000085871.98174.4e. PMID: 14557570.
This may be associated with the “cub” sign in which similar appearances are seen within the pontine tegmentum.
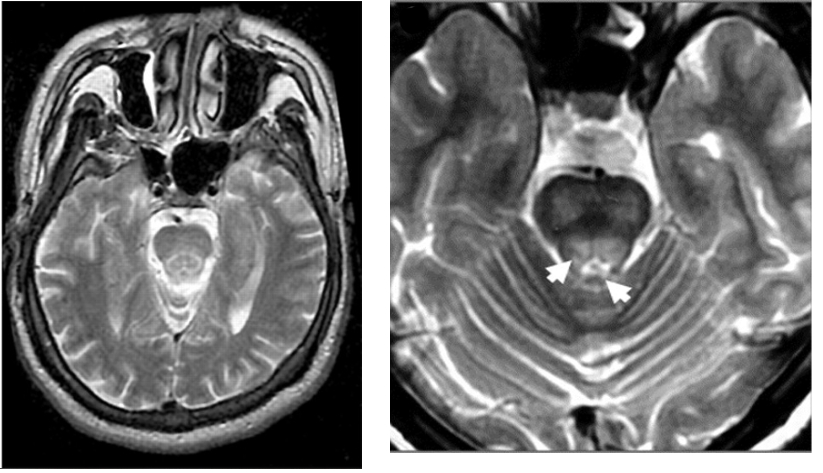
Liebeskind DS, Wong S, Hamilton RH. Faces of the giant panda and her cub: MRI correlates of Wilson's disease. J Neurol Neurosurg Psychiatry. 2003 May;74(5):682. doi: 10.1136/jnnp.74.5.682.
Shivakumar R, Thomas SV. Teaching NeuroImages: face of the giant panda and her cub: MRI correlates of Wilson disease. Neurology. 2009 Mar 17;72(11):e50.

